What Does A Mineralogist Do
Mineralogy [n ane] is a subject of geology specializing in the scientific study of the chemical science, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization.
History [edit]

Early on writing on mineralogy, especially on gemstones, comes from aboriginal Babylonia, the aboriginal Greco-Roman earth, ancient and medieval China, and Sanskrit texts from aboriginal Bharat and the ancient Islamic world.[4] Books on the subject included the Naturalis Historia of Pliny the Elderberry, which non just described many different minerals but likewise explained many of their backdrop, and Kitab al Jawahir (Book of Precious Stones) by Persian scientist Al-Biruni. The High german Renaissance specialist Georgius Agricola wrote works such as De re metallica (On Metals, 1556) and De Natura Fossilium (On the Nature of Rocks, 1546) which began the scientific approach to the subject. Systematic scientific studies of minerals and rocks developed in post-Renaissance Europe.[4] The modernistic study of mineralogy was founded on the principles of crystallography (the origins of geometric crystallography, itself, can be traced back to the mineralogy practiced in the eighteenth and nineteenth centuries) and to the microscopic report of stone sections with the invention of the microscope in the 17th century.[4]
Nicholas Steno first observed the law of continuance of interfacial angles (also known as the first law of crystallography) in quartz crystals in 1669.[v] : iv This was later generalized and established experimentally by Jean-Baptiste 50. Romé de l'Islee in 1783.[6] René Just Haüy, the "father of modern crystallography", showed that crystals are periodic and established that the orientations of crystal faces can exist expressed in terms of rational numbers, as later encoded in the Miller indices.[5] : four In 1814, Jöns Jacob Berzelius introduced a classification of minerals based on their chemical science rather than their crystal structure.[7] William Nicol developed the Nicol prism, which polarizes calorie-free, in 1827–1828 while studying fossilized wood; Henry Clifton Sorby showed that thin sections of minerals could exist identified by their optical backdrop using a polarizing microscope.[five] : four [7] : 15 James D. Dana published his get-go edition of A Organization of Mineralogy in 1837, and in a later edition introduced a chemical nomenclature that is still the standard.[five] : iv [seven] : 15 10-ray diffraction was demonstrated by Max von Laue in 1912, and developed into a tool for analyzing the crystal structure of minerals by the father/son team of William Henry Bragg and William Lawrence Bragg.[five] : 4
More recently, driven by advances in experimental technique (such as neutron diffraction) and bachelor computational ability, the latter of which has enabled extremely accurate atomic-scale simulations of the behaviour of crystals, the science has branched out to consider more than full general problems in the fields of inorganic chemical science and solid-land physics. Information technology, however, retains a focus on the crystal structures commonly encountered in stone-forming minerals (such as the perovskites, dirt minerals and framework silicates). In item, the field has made cracking advances in the understanding of the relationship between the atomic-scale construction of minerals and their part; in nature, prominent examples would exist accurate measurement and prediction of the rubberband properties of minerals, which has led to new insight into seismological behaviour of rocks and depth-related discontinuities in seismograms of the Earth's pall. To this stop, in their focus on the connection betwixt atomic-scale phenomena and macroscopic properties, the mineral sciences (every bit they are now commonly known) display perhaps more of an overlap with materials scientific discipline than any other discipline.
Physical backdrop [edit]


An initial step in identifying a mineral is to examine its physical properties, many of which can exist measured on a hand sample. These can be classified into density (often given as specific gravity); measures of mechanical cohesion (hardness, tenacity, cleavage, fracture, parting); macroscopic visual properties (luster, color, streak, luminescence, diaphaneity); magnetic and electric properties; radioactivity and solubility in hydrogen chloride (HCl).[five] : 97–113 [8] : 39–53
Hardness is determined past comparing with other minerals. In the Mohs scale, a standard gear up of minerals are numbered in order of increasing hardness from one (talc) to 10 (diamond). A harder mineral will scratch a softer, so an unknown mineral tin be placed in this scale, by which minerals; it scratches and which scratch it. A few minerals such as calcite and kyanite have a hardness that depends significantly on management.[nine] : 254–255 Hardness can also be measured on an accented scale using a sclerometer; compared to the absolute scale, the Mohs calibration is nonlinear.[8] : 52
Tenacity refers to the fashion a mineral behaves, when information technology is broken, crushed, bent or torn. A mineral can be brittle, malleable, sectile, ductile, flexible or elastic. An important influence on tenacity is the blazon of chemical bond (east.g., ionic or metallic).[nine] : 255–256
Of the other measures of mechanical cohesion, cleavage is the tendency to interruption along certain crystallographic planes. It is described by the quality (eastward.g., perfect or fair) and the orientation of the plane in crystallographic nomenclature.
Departing is the tendency to break along planes of weakness due to pressure, twinning or exsolution. Where these two kinds of intermission do not occur, fracture is a less orderly course that may be conchoidal (having smooth curves resembling the interior of a shell), fibrous, splintery, hackly (jagged with sharp edges), or uneven.[nine] : 253–254
If the mineral is well crystallized, it will as well have a distinctive crystal addiction (for instance, hexagonal, columnar, botryoidal) that reflects the crystal construction or internal organisation of atoms.[viii] : 40–41 It is besides affected by crystal defects and twinning. Many crystals are polymorphic, having more than one possible crystal structure depending on factors such as force per unit area and temperature.[5] : 66–68 [8] : 126
Crystal structure [edit]
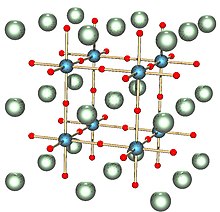
The perovskite crystal structure. The most abundant mineral in the Globe, bridgmanite, has this structure.[ten] Its chemic formula is (Mg,Fe)SiOiii; the ruby spheres are oxygen, the blue spheres silicon and the greenish spheres magnesium or fe.
The crystal structure is the arrangement of atoms in a crystal. Information technology is represented by a lattice of points which repeats a basic pattern, called a unit cell, in 3 dimensions. The lattice can be characterized by its symmetries and by the dimensions of the unit prison cell. These dimensions are represented by three Miller indices.[11] : 91–92 The lattice remains unchanged by certain symmetry operations near any given point in the lattice: reflection, rotation, inversion, and rotary inversion, a combination of rotation and reflection. Together, they make upward a mathematical object called a crystallographic point grouping or crystal grade. There are 32 possible crystal classes. In addition, there are operations that displace all the points: translation, screw axis, and glide plane. In combination with the point symmetries, they class 230 possible infinite groups.[11] : 125–126
Most geology departments take X-ray pulverisation diffraction equipment to analyze the crystal structures of minerals.[8] : 54–55 Ten-rays have wavelengths that are the aforementioned order of magnitude as the distances between atoms. Diffraction, the constructive and destructive interference between waves scattered at dissimilar atoms, leads to distinctive patterns of high and low intensity that depend on the geometry of the crystal. In a sample that is ground to a powder, the X-rays sample a random distribution of all crystal orientations.[12] Pulverisation diffraction can distinguish betwixt minerals that may announced the aforementioned in a hand sample, for example quartz and its polymorphs tridymite and cristobalite.[eight] : 54
Isomorphous minerals of dissimilar compositions have similar powder diffraction patterns, the principal divergence being in spacing and intensity of lines. For example, the NaCl (halite) crystal construction is space group Fm3m; this structure is shared by sylvite (KCl), periclase (MgO), bunsenite (NiO), galena (PbS), alabandite (MnS), chlorargyrite (AgCl), and osbornite (Tin).[9] : 150–151
Chemical elements [edit]

Portable Micro-X-ray fluorescence motorcar
A few minerals are chemical elements, including sulfur, copper, silver, and gold, but the vast majority are compounds. The classical method for identifying limerick is wet chemical analysis, which involves dissolving a mineral in an acid such equally hydrochloric acid (HCl). The elements in solution are then identified using colorimetry, volumetric assay or gravimetric analysis.[9] : 224–225
Since 1960, most chemistry analysis is washed using instruments. 1 of these, diminutive absorption spectroscopy, is similar to wet chemistry in that the sample must nonetheless be dissolved, but it is much faster and cheaper. The solution is vaporized and its assimilation spectrum is measured in the visible and ultraviolet range.[9] : 225–226 Other techniques are 10-ray fluorescence, electron microprobe assay atom probe tomography and optical emission spectrography.[nine] : 227–232
Optical [edit]

In addition to macroscopic properties such as colour or lustre, minerals accept properties that require a polarizing microscope to observe.
Transmitted light [edit]
When low-cal passes from air or a vacuum into a transparent crystal, some of information technology is reflected at the surface and some refracted. The latter is a bending of the light path that occurs considering the speed of light changes as it goes into the crystal; Snell'southward law relates the bending bending to the Refractive index, the ratio of speed in a vacuum to speed in the crystal. Crystals whose betoken symmetry grouping falls in the cubic organisation are isotropic: the alphabetize does not depend on direction. All other crystals are anisotropic: calorie-free passing through them is broken upward into two plane polarized rays that travel at different speeds and refract at different angles.[9] : 289–291
A polarizing microscope is similar to an ordinary microscope, simply it has two aeroplane-polarized filters, a (polarizer) below the sample and an analyzer above it, polarized perpendicular to each other. Light passes successively through the polarizer, the sample and the analyzer. If there is no sample, the analyzer blocks all the light from the polarizer. However, an anisotropic sample will more often than not change the polarization and so some of the light can pass through. Thin sections and powders can be used every bit samples.[nine] : 293–294
When an isotropic crystal is viewed, it appears night because it does not modify the polarization of the light. Withal, when it is immersed in a calibrated liquid with a lower alphabetize of refraction and the microscope is thrown out of focus, a brilliant line called a Becke line appears around the perimeter of the crystal. By observing the presence or absence of such lines in liquids with different indices, the index of the crystal can be estimated, unremarkably to within ± 0.003.[nine] : 294–295
Systematic [edit]

Hanksite, Na22Thou(SO4)9(CO3)2Cl, one of the few minerals that is considered a carbonate and a sulfate
Systematic mineralogy is the identification and classification of minerals by their properties. Historically, mineralogy was heavily concerned with taxonomy of the rock-forming minerals. In 1959, the International Mineralogical Association formed the Commission of New Minerals and Mineral Names to rationalize the nomenclature and regulate the introduction of new names. In July 2006, it was merged with the Committee on Classification of Minerals to form the Commission on New Minerals, Classification, and Nomenclature.[13] In that location are over half-dozen,000 named and unnamed minerals, and nearly 100 are discovered each twelvemonth.[fourteen] The Manual of Mineralogy places minerals in the following classes: native elements, sulfides, sulfosalts, oxides and hydroxides, halides, carbonates, nitrates and borates, sulfates, chromates, molybdates and tungstates, phosphates, arsenates and vanadates, and silicates.[9]
Formation environments [edit]
The environments of mineral germination and growth are highly varied, ranging from slow crystallization at the high temperatures and pressures of igneous melts deep within the Earth's crust to the low temperature atmospheric precipitation from a saline brine at the World's surface.
Various possible methods of germination include:[15]
- sublimation from volcanic gases
- deposition from aqueous solutions and hydrothermal brines
- crystallization from an igneous magma or lava
- recrystallization due to metamorphic processes and metasomatism
- crystallization during diagenesis of sediments
- germination by oxidation and weathering of rocks exposed to the atmosphere or inside the soil environment.
Biomineralogy [edit]
Biomineralogy is a cross-over field between mineralogy, paleontology and biological science. Information technology is the study of how plants and animals stabilize minerals nether biological control, and the sequencing of mineral replacement of those minerals after degradation.[16] It uses techniques from chemical mineralogy, especially isotopic studies, to decide such things as growth forms in living plants and animals[17] [18] every bit well as things like the original mineral content of fossils.[19]
A new approach to mineralogy chosen mineral development explores the co-evolution of the geosphere and biosphere, including the role of minerals in the origin of life and processes as mineral-catalyzed organic synthesis and the selective adsorption of organic molecules on mineral surfaces.[xx] [21]
Mineral ecology [edit]
In 2011, several researchers began to develop a Mineral Evolution Database.[22] This database integrates the crowd-sourced site Mindat.org, which has over 690,000 mineral-locality pairs, with the official IMA list of approved minerals and historic period data from geological publications.[23]
This database makes it possible to utilize statistics to answer new questions, an arroyo that has been chosen mineral environmental. One such question is how much of mineral evolution is deterministic and how much the result of chance. Some factors are deterministic, such equally the chemic nature of a mineral and conditions for its stability; but mineralogy tin likewise be afflicted by the processes that determine a planet'due south limerick. In a 2015 paper, Robert Hazen and others analyzed the number of minerals involving each element as a part of its abundance. They found that Earth, with over 4800 known minerals and 72 elements, has a power police force relationship. The Moon, with only 63 minerals and 24 elements (based on a much smaller sample) has essentially the same relationship. This implies that, given the chemic limerick of the planet, i could predict the more common minerals. All the same, the distribution has a long tail, with 34% of the minerals having been found at only one or two locations. The model predicts that thousands more mineral species may expect discovery or have formed and and so been lost to erosion, burial or other processes. This implies a office of chance in the germination of rare minerals occur.[24] [25] [26] [27]
In another use of big information sets, network theory was practical to a dataset of carbon minerals, revealing new patterns in their diverseness and distribution. The analysis can testify which minerals tend to coexist and what conditions (geological, physical, chemical and biological) are associated with them. This information can be used to predict where to look for new deposits and even new mineral species.[28] [29] [30]
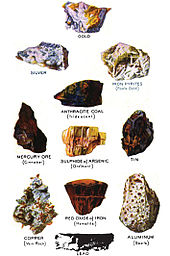
A colour chart of some raw forms of commercially valuable metals.[31]
Uses [edit]
Minerals are essential to various needs within human society, such equally minerals used as ores for essential components of metal products used in various commodities and machinery, essential components to building materials such as limestone, marble, granite, gravel, drinking glass, plaster, cement, etc.[15] Minerals are as well used in fertilizers to enrich the growth of agronomical crops.

A modest collection of mineral samples, with cases. Labels in Russian.
Collecting [edit]
Mineral collecting is also a recreational study and drove hobby, with clubs and societies representing the field.[32] [33] Museums, such every bit the Smithsonian National Museum of Natural History Hall of Geology, Gems, and Minerals, the Natural History Museum of Los Angeles County, the Carnegie Museum of Natural History, the Natural History Museum, London, and the private Mim Mineral Museum in Beirut, Lebanon,[34] [35] have pop collections of mineral specimens on permanent display.[36]
Encounter also [edit]
- List of minerals
- List of minerals recognized by the International Mineralogical Association
- List of mineralogists
- List of publications in mineralogy
- Mineral collecting
- Mineral physics
- Metallurgy
- Petrology
Notes [edit]
- ^ Normally pronounced [1] [2] due to the common phonological procedure of anticipatory assimilation, particularly in North-American but also in United kingdom English. Nevertheless, even modern descriptive United kingdom of great britain and northern ireland dictionaries tend to record only the spelling pronunciation , sometimes even while their audio file instead has the assimilated pronunciation, as in the instance of the Collins Dictionary.[2] [ failed verification ]
References [edit]
- ^ "mineralogy". The American Heritage Dictionary of the English Linguistic communication (5th ed.). HarperCollins. Retrieved 2017-ten-19 .
- ^ a b "mineralogy". CollinsDictionary.com. HarperCollins. Retrieved 2017-10-19 .
- ^ "NASA Instrument Inaugurates iii-D Moon Imaging". JPL. Retrieved 19 December 2008.
- ^ a b c Needham, Joseph (1959). Science and civilisation in Cathay . Cambridge: Cambridge University Press. pp. 637–638. ISBN978-0521058018.
- ^ a b c d due east f m Nesse, William D. (2012). Introduction to mineralogy (2nd ed.). New York: Oxford University Press. ISBN978-0199827381.
- ^ "Constabulary of the constancy of interfacial angles". Online dictionary of crystallography. International Union of Crystallography. 24 August 2014. Retrieved 22 September 2015.
- ^ a b c Rafferty, John P. (2012). Geological sciences (1st ed.). New York: Britannica Educational Pub. in clan with Rosen Educational Services. pp. 14–15. ISBN9781615304950.
- ^ a b c d e f Klein, Cornelis; Philpotts, Anthony R. (2013). Earth materials : introduction to mineralogy and petrology. New York: Cambridge University Printing. ISBN9780521145213.
- ^ a b c d east f yard h i j g Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. ISBN047157452X.
- ^ Abrupt, T. (27 November 2014). "Bridgmanite – named at last". Science. 346 (6213): 1057–1058. Bibcode:2014Sci...346.1057S. doi:10.1126/science.1261887. PMID 25430755. S2CID 206563252.
- ^ a b Ashcroft, Neil W.; Mermin, N. David (1977). Solid state physics (27. repr. ed.). New York: Holt, Rinehart and Winston. ISBN9780030839931.
- ^ Dinnebier, Robert Eastward.; Billinge, Simon J.L. (2008). "1. Principles of powder diffraction". In Dinnebier, Robert E.; Billinge, Simon J.L. (eds.). Pulverisation diffraction : theory and practice (Repr. ed.). Cambridge: Royal Society of Chemical science. pp. 1–nineteen. ISBN9780854042319.
- ^ Parsons, Ian (October 2006). "International Mineralogical Association". Elements. 2 (6): 388. doi:10.2113/gselements.two.6.388.
- ^ Higgins, Michael D.; Smith, Dorian G. W. (Oct 2010). "A demography of mineral species in 2010". Elements. 6 (5): 346.
- ^ a b Moses, Alfred J. (1918–1920). "Mineralogy". In Ramsdell, Lewis Southward. (ed.). Encyclopedia Americana: International Edition. Vol. 19. New York: Americana Corporation. pp. 164–168.
- ^ Scurfield, Gordon (1979). "Wood Petrifaction: an aspect of biomineralogy". Australian Journal of Botany. 27 (iv): 377–390. doi:x.1071/bt9790377.
- ^ Christoffersen, 1000.R.; Balic-Zunic, T.; Pehrson, S.; Christoffersen, J. (2001). "Kinetics of Growth of Columnar Triclinic Calcium Pyrophosphate Dihydrate Crystals". Crystal Growth & Design. 1 (six): 463–466. doi:x.1021/cg015547j.
- ^ Chandrajith, R.; Wijewardana, G.; Dissanayake, C.B.; Abeygunasekara, A. (2006). "Biomineralogy of homo urinary calculi (kidney stones) from some geographic regions of Sri Lanka". Ecology Geochemistry and Health. 28 (four): 393–399. doi:10.1007/s10653-006-9048-y. PMID 16791711. S2CID 24627795.
- ^ Lowenstam, Heitz A (1954). "Environmental relations of modification compositions of certain carbonate secreting marine invertebrates". Proceedings of the National Academy of Sciences of the United states of america of America. 40 (1): 39–48. Bibcode:1954PNAS...40...39L. doi:10.1073/pnas.40.1.39. PMC527935. PMID 16589423.
- ^ Amos, Jonathan (13 Feb 2016). "Earth's rarest minerals catalogued". BBC News . Retrieved 17 September 2016.
- ^ Hazen, Robert M.; Papineau, Dominic; Bleeker, Wouter; Downs, Robert T.; Ferry, John Thousand.; et al. (Nov–Dec 2008). "Mineral Evolution". American Mineralogist. 93 (11–12): 1693–1720. Bibcode:2008AmMin..93.1693H. doi:10.2138/am.2008.2955. S2CID 27460479.
- ^ Hazen, R. G.; Bekker, A.; Bish, D. L.; Bleeker, W.; Downs, R. T.; Farquhar, J.; Ferry, J. Chiliad.; Grew, E. Southward.; Knoll, A. H.; Papineau, D.; Ralph, J. P.; Sverjensky, D. A.; Valley, J. W. (24 June 2011). "Needs and opportunities in mineral evolution inquiry". American Mineralogist. 96 (seven): 953–963. Bibcode:2011AmMin..96..953H. doi:10.2138/am.2011.3725. S2CID 21530264.
- ^ Aureate, Joshua; Pires, Alexander J.; Hazenj, Robert M.; Downs, Robert T.; Ralph, Jolyon; Meyer, Michael Bruce (2016). Building the mineral development database: implications for future big data analysis. GSA Annual Meeting. Denver, Colorado. doi:10.1130/abs/2016AM-286024.
- ^ Hazen, Robert M.; Grew, Edward S.; Downs, Robert T.; Golden, Joshua; Hystad, Grethe (March 2015). "Mineral ecology: Chance and necessity in the mineral variety of terrestrial planets". The Canadian Mineralogist. 53 (2): 295–324. doi:10.3749/canmin.1400086. S2CID 10969988.
- ^ Hazen, Robert. "Mineral Ecology". Carnegie Science . Retrieved 15 May 2018.
- ^ Kwok, Roberta (11 August 2015). "Is Mineral Evolution Driven by Chance?". Quanta Magazine . Retrieved 11 August 2018.
- ^ Kwok, Roberta (16 Baronial 2015). "How Life and Luck Inverse Earth'southward Minerals". Wired . Retrieved 24 August 2018.
- ^ Oleson, Timothy (1 May 2018). "Data-driven discovery reveals Earth'due south missing minerals". Globe Magazine. American Geosciences Constitute. Retrieved 26 Baronial 2018.
- ^ Hooper, Joel (two August 2017). "Data mining: How digging through big information tin turn upwards new". Cosmos . Retrieved 26 August 2018.
- ^ Rogers, Nala (one August 2017). "How Math Can Assistance Geologists Discover New Minerals". Inside Science . Retrieved 26 August 2018.
- ^ The Encyclopedia Americana. New York: Encyclopedia Americana Corp. 1918–1920. plate opposite p. 166.
- ^ "Collector'south Corner". The Mineralogical Society of America. Retrieved 2010-05-22 .
- ^ "The American Federation of Mineral Societies". Retrieved 2010-05-22 .
- ^ Wilson, W (2013). "The Opening of the Mim Mineral Museum in Beirut, Lebanon". The Mineralogical Record. 45 (ane): 61–83.
- ^ Lyckberg, Peter (xvi October 2013). "The MIM Museum opening, Lebanon". Mindat.org. Retrieved 19 October 2017.
- ^ "Gems and Minerals". Natural History Museum of Los Angeles. Retrieved 2010-05-22 .
Farther reading [edit]
- Gribble, C.D.; Hall, A.J. (1993). Optical Mineralogy: Principles And Practise. London: CRC Printing. ISBN9780203498705.
- Harrell, James A. (2012). "Mineralogy". In Bagnall, Roger S.; Brodersen, Kai; Champion, Craige B.; Erskine, Andrew (eds.). The encyclopedia of ancient history. Malden, MA: Wiley-Blackwell. doi:10.1002/9781444338386.wbeah21217. ISBN9781444338386.
- Hazen, Robert M. (1984). "Mineralogy: A historical review" (PDF). Journal of Geological Education. 32 (five): 288–298. Bibcode:1984JGeoE..32..288H. doi:10.5408/0022-1368-32.5.288. Retrieved 27 September 2017.
- Laudan, Rachel (1993). From mineralogy to geology : the foundations of a science, 1650-1830 (Pbk. ed.). Chicago: University of Chicago Press. ISBN9780226469478.
- Oldroyd, David (1998). Sciences of the earth : studies in the history of mineralogy and geology. Aldershot: Ashgate. ISBN9780860787709.
- Perkins, Dexter (2014). Mineralogy. Pearson Higher Ed. ISBN9780321986573.
- Rapp, George R. (2002). Archaeomineralogy. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN9783662050057.
- Tisljar, S.K. Haldar, Josip (2013). Introduction to mineralogy and petrology. Burlington: Elsevier Science. ISBN9780124167100.
- Wenk, Hans-Rudolf; Bulakh, Andrey (2016). Minerals: Their Constitution and Origin. Cambridge University Press. ISBN9781316425282.
- Whewell, William (2010). "Book XV. History of Mineralogy". History of the Inductive Sciences: From the Primeval to the Nowadays Times. Cambridge Academy Printing. pp. 187–252. ISBN9781108019262.
External links [edit]
![]()
Wikimedia Eatables has media related to Mineralogy.
![]()
Wikisource has original works on the topic: Mineralogy
- The Virtual Museum of the History of Mineralogy
Associations [edit]
- American Federation of Mineral Societies
- French Society of Mineralogy and Crystallography
- Geological Society of America
- High german Mineralogical Society
- International Mineralogical Clan
- Italian Mineralogical and Petrological Society
- Mineralogical Association of Canada
- Mineralogical Guild of Great Britain and Ireland
- Mineralogical Guild of America
What Does A Mineralogist Do,
Source: https://en.wikipedia.org/wiki/Mineralogy
Posted by: pricedrabland1987.blogspot.com


0 Response to "What Does A Mineralogist Do"
Post a Comment